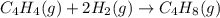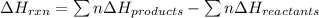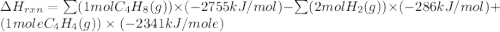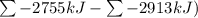Chemistry, 22.11.2019 00:31 montrellgoodman5890
Ombustion reactions involve reacting a substance with oxygen. when compounds containing carbon and hydrogen are combusted, carbon dioxide and water are the products. using the enthalpies of combustion for c4h4 (-2341 kj/mol), c4h8 (-2755 kj/mol), and h2 (-286 kj/mol), calculate δh for the reaction.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 09:00
A2-kg bowling ball is 1 meter off the ground on a post when it falls. just before it reaches the ground,its traveling 4.4 m/s. assuming that there is no air resistant, which statement is true a. the initial potential energy is less then the final kinetic energy b. the mechanical energy is not conserved c. the mechanical energy is conserved d. the initial potential energy is greater than the final kinetic energy
Answers: 3

Chemistry, 23.06.2019 09:20
1) a. water molecule breaks up into hydrogen and oxygen on passing electricity. does this involve breaking intermolecular or intramolecular forces of attraction. explain b. on boiling water changes to water vapor. does this involve breaking intermolecular or intramolecular forces of attraction. explain methanol evaporates faster than water. contrast the intermolecular forces and the vapor pressures of methanol and water?
Answers: 2
You know the right answer?
Ombustion reactions involve reacting a substance with oxygen. when compounds containing carbon and h...
Questions


Computers and Technology, 06.09.2019 22:30

History, 06.09.2019 22:30

History, 06.09.2019 22:30


Mathematics, 06.09.2019 22:30

English, 06.09.2019 22:30


Biology, 06.09.2019 22:30



History, 06.09.2019 22:30

History, 06.09.2019 22:30

Physics, 06.09.2019 22:30


History, 06.09.2019 22:30


Mathematics, 06.09.2019 22:30

English, 06.09.2019 22:30