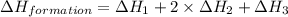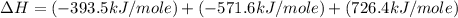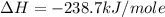Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
Given the following heats of combustion. ch3oh(l) + 3/2 o2(g) co2(g) + 2 h2o(l) δh°rxn = -726.4 kj c...
Questions





Mathematics, 04.08.2019 19:00



English, 04.08.2019 19:00



Health, 04.08.2019 19:00

Social Studies, 04.08.2019 19:00

History, 04.08.2019 19:00

Mathematics, 04.08.2019 19:00





History, 04.08.2019 19:00

Mathematics, 04.08.2019 19:00

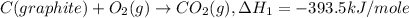 ..[1]
..[1]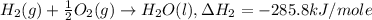 ..[2]
..[2]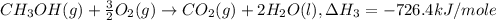 ..[3]
..[3]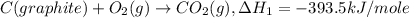 ..[1]
..[1]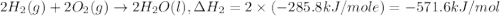 ..[2]
..[2]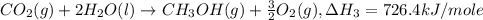 [3]
[3]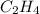 will be,
will be,