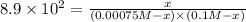Chemistry, 22.11.2019 01:31 ellenaschool
Potassium thiocyanate, kscn, is often used to detect the presence of fe3+ ions in solution through the formation of the red fe(h2o)5scn2+ (or, more simply, fescn2+). what is [fe3+] when 0.700 l each of 0.00150 m fe(no3)3 and 0.200 m kscn are mixed? kf of fescn2+ = 8.9 × 102. enter your answer in scientific notation. report your final answer to two significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1
You know the right answer?
Potassium thiocyanate, kscn, is often used to detect the presence of fe3+ ions in solution through t...
Questions


History, 25.11.2020 22:10

Physics, 25.11.2020 22:10

Mathematics, 25.11.2020 22:10

English, 25.11.2020 22:10


Biology, 25.11.2020 22:10




Mathematics, 25.11.2020 22:10






Mathematics, 25.11.2020 22:10


English, 25.11.2020 22:10

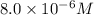 .
.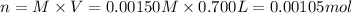
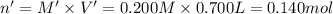
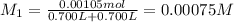
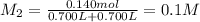
![Fe^{3+}+SCN^-\rightleftharpoons [Fe(SCN)]^{2+}](/tpl/images/0385/5120/942bc.png)
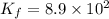
![K_f=\frac{[[Fe(SCN)]^{2+}]}{[[Fe^{3+}]][SCN^{-}]}](/tpl/images/0385/5120/f34ae.png)