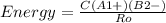Chemistry, 22.11.2019 01:31 makaylahunt
An ionic bond is formed between a cation a1 and an anion b2. how would the energy of the ionic bond [see equation (9.2)] be affected by the following changes? (a) doubling the radius of a1, (b) tripling the charge on a1, (c) doubling the charges on a1 and b2, (d) decreasing the radii of a1 and b2 to half their original values.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1


Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
An ionic bond is formed between a cation a1 and an anion b2. how would the energy of the ionic bond...
Questions

Computers and Technology, 13.03.2022 16:30

Engineering, 13.03.2022 16:30


History, 13.03.2022 16:30


Mathematics, 13.03.2022 16:40


Mathematics, 13.03.2022 16:40



Mathematics, 13.03.2022 16:40

Mathematics, 13.03.2022 16:40






Computers and Technology, 13.03.2022 16:50