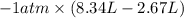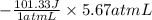Chemistry, 22.11.2019 02:31 cassandramanuel
Asample of an ideal gas in a cylinder of volume 2.67 l at 298 k and 2.81 atm expands to 8.34 l by two different pathways. path a is an isothermal, reversible expansion. path b has two steps. in the first step, the gas is cooled at constant volume to 1.00 atm. in the second step, the gas is heated and allowed to expand against a constant external pressure of 1.00 atm until the final volume is 8.34 l. calculate the work for path a and b.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2
You know the right answer?
Asample of an ideal gas in a cylinder of volume 2.67 l at 298 k and 2.81 atm expands to 8.34 l by tw...
Questions


Biology, 23.02.2021 19:50


Social Studies, 23.02.2021 19:50


History, 23.02.2021 19:50


History, 23.02.2021 19:50





Chemistry, 23.02.2021 19:50

Physics, 23.02.2021 19:50

Physics, 23.02.2021 19:50


Mathematics, 23.02.2021 19:50

Mathematics, 23.02.2021 19:50


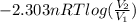
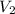 = 8.34 L,
= 8.34 L, 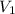 = 2.67 L
= 2.67 L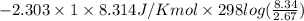
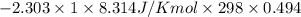
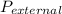 = 1.00 atm.
= 1.00 atm.