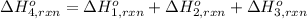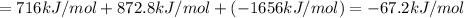Chemistry, 22.11.2019 03:31 CyberSongWriter
Using the following information and the fact that the average c―h bond enthalpy is 414 kj/mol, estimate the standard enthalpy of formation of methane (ch4). c(s) → c(g) δh o rxn = 716 kj/mol 2h2(g) → 4h(g) δh o rxn = 872.8 kj/mol

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Which of the following statements concerning the influence of culture on ethnic identity formation is accurate? a. one will reject ethnic identity if cultural stereotypes are encountered. b. if one’s ethnic city is different from the dominant cultural group, then one’s ethnic identity you will become weekend. c. if an the ethnic group is excepted by dominant culture, then ethnic identity formation can be a difficult process. d. similarity to the dominant culture can determine how easy it is for one to except ethnic differences.
Answers: 2

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
Using the following information and the fact that the average c―h bond enthalpy is 414 kj/mol, estim...
Questions


Mathematics, 05.05.2021 21:50

Mathematics, 05.05.2021 21:50

Mathematics, 05.05.2021 21:50

Mathematics, 05.05.2021 21:50

Mathematics, 05.05.2021 21:50


Mathematics, 05.05.2021 21:50

Chemistry, 05.05.2021 21:50

Mathematics, 05.05.2021 21:50

Mathematics, 05.05.2021 21:50

Mathematics, 05.05.2021 21:50




Biology, 05.05.2021 21:50




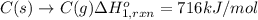 ...[1]
...[1]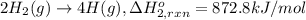 ...[2]
...[2]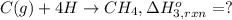 ...[3]
...[3]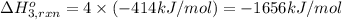
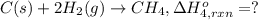 ...[4]
...[4]