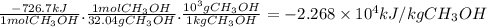Chemistry, 22.11.2019 03:31 playaajosh
Hydrogen and methanol have both been proposed as alternatives to hydrocarbon fuels. write balanced reactions for the complete combustion of hydrogen and methanol (identify phases) and use standard enthalpies of formation to calculate the amount of heat released per kilogram of the fuel (kj/kg). which fuel contains the most energy in the least mass? how does the energy of these fuels compare to that of octane (c8h18) (amount of heat released by octane (kj/

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
Hydrogen and methanol have both been proposed as alternatives to hydrocarbon fuels. write balanced r...
Questions




Mathematics, 11.06.2020 02:57









Mathematics, 11.06.2020 02:57

Social Studies, 11.06.2020 02:57





Social Studies, 11.06.2020 02:57