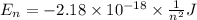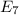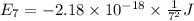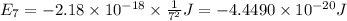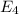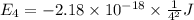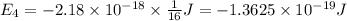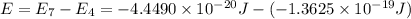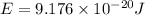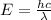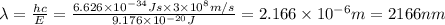Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2


Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1
You know the right answer?
Calculate the wavelength, in nanometers, of the light emitted by a hydrogen atom when its electron f...
Questions

Mathematics, 19.03.2021 21:20


Mathematics, 19.03.2021 21:20


Mathematics, 19.03.2021 21:20

Mathematics, 19.03.2021 21:20


Mathematics, 19.03.2021 21:20

Physics, 19.03.2021 21:20

Mathematics, 19.03.2021 21:20



Mathematics, 19.03.2021 21:20


Mathematics, 19.03.2021 21:20

Mathematics, 19.03.2021 21:20


Mathematics, 19.03.2021 21:20


Mathematics, 19.03.2021 21:20