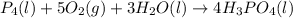Phosphoric acid, which is commonly used as rust inhibitor, food additive and etching agent for dental and orthopedic use, can be synthesized using a two-step thermal process. in the first step, phosphorus and oxygen react to form diphosphorus pentoxide: p4(l)+5o2(g-2 p20s(g) in the second step, diphosphorus pentoxide and water react to form phosphoric acld p20(9)+3 h200 2h, po40) write the net chemical equation for the production of phosphoric acid from phosphorus, oxygen and water. be sure your equation is balanced.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1
You know the right answer?
Phosphoric acid, which is commonly used as rust inhibitor, food additive and etching agent for denta...
Questions




Mathematics, 15.05.2021 22:10

Mathematics, 15.05.2021 22:10

Mathematics, 15.05.2021 22:10

SAT, 15.05.2021 22:10

Social Studies, 15.05.2021 22:10

Mathematics, 15.05.2021 22:10


Computers and Technology, 15.05.2021 22:10



Mathematics, 15.05.2021 22:10


Arts, 15.05.2021 22:10




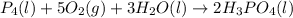
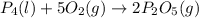 ......(1)
......(1)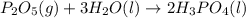 .......(2)
.......(2)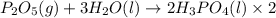
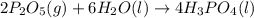 .......(3)
.......(3)