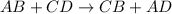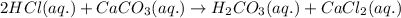Chemistry, 22.11.2019 04:31 morgannwaldrupp
To measure the amount of calcium carbonate (caco) in a seashell, an analytical chemist crushes a 2.100 g sample of the shell to a fine powder and titrates it to the endpoint with 119. ml of 0.300 m hydrogen chloride (hci) solution. the balanced chemical equation for the reaction is 2hci(aq) + co, (aq) ? h2co3(aq) + 2a (aq). what kind of reaction is this?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2


Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1
You know the right answer?
To measure the amount of calcium carbonate (caco) in a seashell, an analytical chemist crushes a 2.1...
Questions

Mathematics, 05.11.2020 23:00

Mathematics, 05.11.2020 23:00


History, 05.11.2020 23:00

Social Studies, 05.11.2020 23:00





Mathematics, 05.11.2020 23:00








English, 05.11.2020 23:00


Mathematics, 05.11.2020 23:00