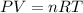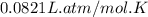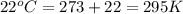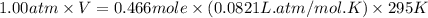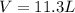Automobile airbags contain solid sodium azide, nan3, that reacts to produce nitrogen gas when heated, thus inflating the bag. 2nan3(s) > 2na(s) + 3n2(g) calculate the value of w (work) for the following system if 20.2 g of nan3 reacts completely at 1.00 atm and 22 degrees

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3
You know the right answer?
Automobile airbags contain solid sodium azide, nan3, that reacts to produce nitrogen gas when heated...
Questions


History, 31.08.2019 15:30



Mathematics, 31.08.2019 15:30

Mathematics, 31.08.2019 15:30

Social Studies, 31.08.2019 15:30



Health, 31.08.2019 15:30






Mathematics, 31.08.2019 15:30

Chemistry, 31.08.2019 15:30

English, 31.08.2019 15:30

Chemistry, 31.08.2019 15:30

Mathematics, 31.08.2019 15:30

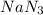
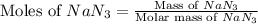
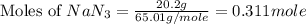
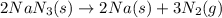
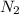
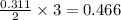 moles of
moles of