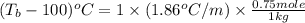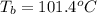Chemistry, 22.11.2019 05:31 ivanyeli4520
Ethylene glycol, c2h6o2, a nonelectrolyte, is added to the water in a radiator to give a solution containing 0.75 mole of ethylene glycol in 1 kg of water (solvent). what is the boiling point of the solution?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1


Chemistry, 22.06.2019 05:40
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2
You know the right answer?
Ethylene glycol, c2h6o2, a nonelectrolyte, is added to the water in a radiator to give a solution co...
Questions


Mathematics, 24.03.2021 20:10

Mathematics, 24.03.2021 20:10


Biology, 24.03.2021 20:10

Mathematics, 24.03.2021 20:10

Business, 24.03.2021 20:10




Mathematics, 24.03.2021 20:10


Mathematics, 24.03.2021 20:10

Mathematics, 24.03.2021 20:10

History, 24.03.2021 20:10

Mathematics, 24.03.2021 20:10



History, 24.03.2021 20:10

Mathematics, 24.03.2021 20:10

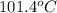
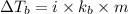
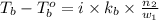
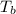 = boiling point of solution = ?
= boiling point of solution = ?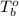 = boiling point of water =
= boiling point of water = 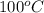
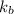 = boiling point constant of water =
= boiling point constant of water = 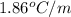
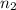 = moles of solute (ethylene glycol) = 0.75 mole
= moles of solute (ethylene glycol) = 0.75 mole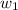 = mass of solvent (water) = 1 kg
= mass of solvent (water) = 1 kg