Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2


Chemistry, 23.06.2019 06:10
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1

Chemistry, 23.06.2019 10:30
Which of the following pairs of elements is most likely to form an ionic compound? a oxygen and fluorine b sodium and aluminum c calcium and chlorine d nitrogen and sulfur
Answers: 1
You know the right answer?
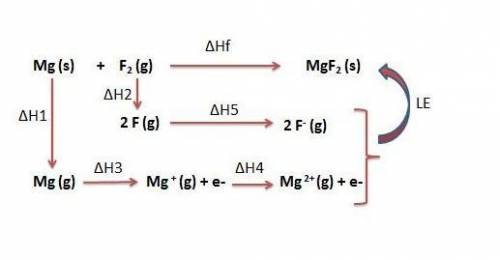
Use the following to calculate h°lattice of mgf2. mg(s) mg(g) h° = 148 kj f2(g) 2 f(g) h° = 159 kj m...
Questions







English, 25.03.2020 06:43

English, 25.03.2020 06:43

Geography, 25.03.2020 06:43


Mathematics, 25.03.2020 06:43

English, 25.03.2020 06:43

History, 25.03.2020 06:43




Mathematics, 25.03.2020 06:43