
Chemistry, 22.11.2019 07:31 reddmeans6
What is the concentration of nacl in solution made by mixing 25.0 ml of .100 m nacl with 75.0 ml of .175 m nacl

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2


Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
What is the concentration of nacl in solution made by mixing 25.0 ml of .100 m nacl with 75.0 ml of...
Questions

Mathematics, 26.06.2019 02:10


Mathematics, 26.06.2019 02:10

Mathematics, 26.06.2019 02:10


Advanced Placement (AP), 26.06.2019 02:10


Mathematics, 26.06.2019 02:10

History, 26.06.2019 02:20

English, 26.06.2019 02:20

English, 26.06.2019 02:20

English, 26.06.2019 02:20

Mathematics, 26.06.2019 02:20

Mathematics, 26.06.2019 02:20






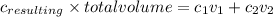 .
.

