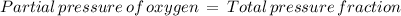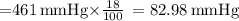Chemistry, 22.11.2019 09:31 nevaehkirk1997
The atmospheric pressure at this altitude is 461 mmhg. assuming that the atmosphere is 18% oxygen (by volume), calculate the partial pressure of o2.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
The atmospheric pressure at this altitude is 461 mmhg. assuming that the atmosphere is 18% oxygen (b...
Questions

Geography, 18.10.2020 01:01

Geography, 18.10.2020 01:01


Chemistry, 18.10.2020 01:01

Physics, 18.10.2020 01:01

Mathematics, 18.10.2020 01:01



English, 18.10.2020 01:01

Spanish, 18.10.2020 01:01

Mathematics, 18.10.2020 01:01

History, 18.10.2020 01:01

Arts, 18.10.2020 01:01

History, 18.10.2020 01:01


English, 18.10.2020 01:01



Mathematics, 18.10.2020 01:01