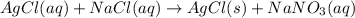Chemistry, 22.11.2019 19:31 jadabecute3739
 + nacl(aq) \rightarrow agcl(s)\ +\ nano _3(aq)) in an experiment a student mixes a 50.0 ml sample of 0.100 m agno₃ (aq) with a 50.0 ml sample of 0.100 m nacl(aq) at 20.0°c in a coffee-cup calorimeter. which of the following is the enthalpy change of the precipitation reaction represented above if the final temperature of the mixture is 21.0°c? (assume that the total mass of the mixture is 100. g and that the specific heat capacity of the mixture is 4.2 j/(g.° (a) -84 kj/mol, (b) -0.42 kj/mol (c) 0.42 kj/molu (d) 84 kj/molpx
in an experiment a student mixes a 50.0 ml sample of 0.100 m agno₃ (aq) with a 50.0 ml sample of 0.100 m nacl(aq) at 20.0°c in a coffee-cup calorimeter. which of the following is the enthalpy change of the precipitation reaction represented above if the final temperature of the mixture is 21.0°c? (assume that the total mass of the mixture is 100. g and that the specific heat capacity of the mixture is 4.2 j/(g.° (a) -84 kj/mol, (b) -0.42 kj/mol (c) 0.42 kj/molu (d) 84 kj/molpx

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
[tex]agno _3(aq) + nacl(aq) \rightarrow agcl(s)\ +\ nano _3(aq)[/tex]in an experiment a student mixe...
Questions



Mathematics, 16.11.2019 07:31

Computers and Technology, 16.11.2019 07:31


Physics, 16.11.2019 07:31


Mathematics, 16.11.2019 07:31

Mathematics, 16.11.2019 07:31


Physics, 16.11.2019 07:31



Mathematics, 16.11.2019 07:31




Mathematics, 16.11.2019 07:31

Chemistry, 16.11.2019 07:31

Mathematics, 16.11.2019 07:31


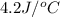
 = final temperature =
= final temperature = 
 = initial temperature =
= initial temperature = 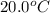



 = enthalpy change = ?
= enthalpy change = ?