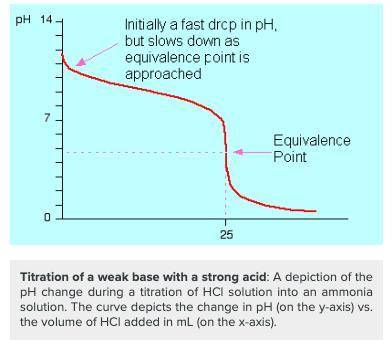
Chemistry, 22.11.2019 20:31 alexfvdsdfgfd8151
Which of the following would you identify a titration curve that involved a strong acid titrated by a weak base?
a. the ph at the equivalence point is lower than 7.
b. the ph at the equivalence point is higher than 7.
c. the titration curve begins at a higher ph and ends at a lower ph.
d. there is a rapid change in ph near the equivalence point (ph = 7).

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:10
How does chemistry affect our world? a. chemicals makes our world more polluted. b. chemicals keeps us healthy. c. chemicals can or hurt our world. d. chemicals make our world safe to live in.
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
Which of the following would you identify a titration curve that involved a strong acid titrated by...
Questions


Mathematics, 25.11.2019 11:31



Arts, 25.11.2019 11:31

Biology, 25.11.2019 11:31

History, 25.11.2019 11:31

Biology, 25.11.2019 11:31


English, 25.11.2019 11:31



Health, 25.11.2019 11:31

Mathematics, 25.11.2019 11:31




Social Studies, 25.11.2019 11:31


History, 25.11.2019 11:31