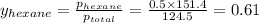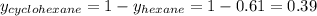Determine the mole fractions of each component in the vapor phase of the vapor in equilibrium with a 1: 1 molar ratio of hexane (c6h14) and cyclohexane (c6h12). the equilibrium vapor pressures of hexane and cyclohexane are equal to 151.4 torr and 97.6 torr respectively

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2


Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
Determine the mole fractions of each component in the vapor phase of the vapor in equilibrium with a...
Questions


Mathematics, 04.08.2020 06:01

Mathematics, 04.08.2020 06:01


Mathematics, 04.08.2020 06:01


Spanish, 04.08.2020 06:01



Mathematics, 04.08.2020 06:01

Geography, 04.08.2020 06:01

Mathematics, 04.08.2020 06:01



Physics, 04.08.2020 06:01

Social Studies, 04.08.2020 06:01

Mathematics, 04.08.2020 06:01

Mathematics, 04.08.2020 06:01

Biology, 04.08.2020 06:01

Biology, 04.08.2020 06:01

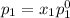 and
and 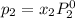
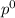 = pressure in the pure state
= pressure in the pure state
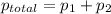
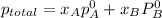
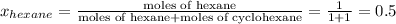 ,
, 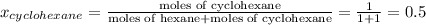 ,
, 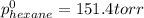
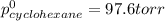
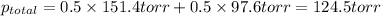
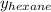 = mole fraction of hexane in vapor phase
= mole fraction of hexane in vapor phase