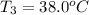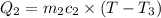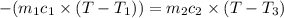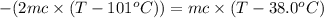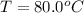Chemistry, 23.11.2019 00:31 sidallen05
One piece of copper jewelry at 101°c has twice the mass of another piece, which is at 38.0°c. both pieces are placed inside a calorimeter of negligible heat capacity. what is the final temperature inside the calorimeter (c of copper=0.387 j/g. k)?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3
You know the right answer?
One piece of copper jewelry at 101°c has twice the mass of another piece, which is at 38.0°c. both p...
Questions


History, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00


English, 21.05.2021 01:00


Social Studies, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00

English, 21.05.2021 01:00


English, 21.05.2021 01:00

English, 21.05.2021 01:00


Mathematics, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00




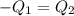
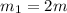
 = 0.387 J/g.K
= 0.387 J/g.K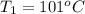
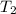 =T
=T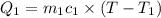
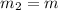
 = 0.387 J/g.K
= 0.387 J/g.K