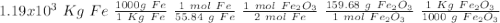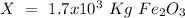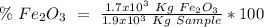Chemistry, 23.11.2019 00:31 mjweed3381
One of the reactions that occurs in a blast furnace, in which iron ore is converted to cast iron, is fe2o3 + 3co → 2fe + 3co2 suppose that 1.19 × 103 kg of fe is obtained from a 1.90 × 103 kg sample of fe2o3. assuming that the reaction goes to completion, what is the percent purity of fe2o3 in the original sample?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
One of the reactions that occurs in a blast furnace, in which iron ore is converted to cast iron, is...
Questions


Mathematics, 15.07.2019 08:00


Mathematics, 15.07.2019 08:00




Health, 15.07.2019 08:00



Business, 15.07.2019 08:00

Biology, 15.07.2019 08:00


Mathematics, 15.07.2019 08:00


Mathematics, 15.07.2019 08:00

Mathematics, 15.07.2019 08:00

Mathematics, 15.07.2019 08:00

Mathematics, 15.07.2019 08:00

Mathematics, 15.07.2019 08:00

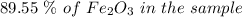
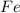 to grams of
to grams of 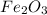 .
.