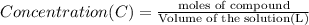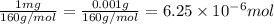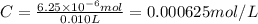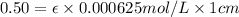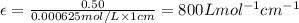Chemistry, 23.11.2019 00:31 chelseychew32
One milligram of a compound of molecular weight 160 is dissolved in 10 ml of ethanol, and the solution is poured into a 1-cm uv cell. the uv spectrum is taken, and there is an absorption at = 247 nm. the maximum absorbance at 247 nm is 0.50. calculate the value of for this absorption.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2
You know the right answer?
One milligram of a compound of molecular weight 160 is dissolved in 10 ml of ethanol, and the soluti...
Questions

Mathematics, 09.10.2019 07:10


English, 09.10.2019 07:10









English, 09.10.2019 07:10

Mathematics, 09.10.2019 07:10

History, 09.10.2019 07:10

Mathematics, 09.10.2019 07:10

Mathematics, 09.10.2019 07:10


Mathematics, 09.10.2019 07:10


Geography, 09.10.2019 07:10

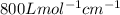 .
.
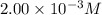
 = molar absorptivity coefficient
= molar absorptivity coefficient