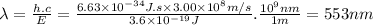The lines on absorption atomic spectra correspond to energies needed for electrons to be excited from a lower energy level to a higher energy level. assume that the energy needed for an electron in 2p orbital in an o atom to jump to 3s orbital is 3.6*10^-19 j, what is its wavelength of the line atomic spectra in nanometer (nm)?
note: use whole numbers and 3 sig figs, or no decimal place.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 23.06.2019 09:10
A155.0 9 piece of copper at 182 °c is dropped into 2500 g of water at 23.9 °c. (the specific heat of copper is 0.385 1/9°c.) calculate the final temperature of the mixture. (assume no heat loss to the surroundings.)
Answers: 2

Chemistry, 23.06.2019 10:00
Why sncl2 is solid while sncl4 is liquid at room temprature explain it in easy way
Answers: 1
You know the right answer?
The lines on absorption atomic spectra correspond to energies needed for electrons to be excited fro...
Questions


History, 06.05.2020 03:15


Chemistry, 06.05.2020 03:15







Mathematics, 06.05.2020 03:15

Physics, 06.05.2020 03:15

Arts, 06.05.2020 03:15

History, 06.05.2020 03:15




History, 06.05.2020 03:15


Business, 06.05.2020 03:16