
For a beaker containing the reaction below that is at equilibrium, using lechatlier's principle, predict which way the equilibrium conditions will shift (type in left, right, or no change) if silver chloride was added to a beaker.
agno3 (aq) + nacl (aq) → agcl (s) + nano3 (aq)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1
You know the right answer?
For a beaker containing the reaction below that is at equilibrium, using lechatlier's principle, pre...
Questions

Geography, 12.01.2021 01:30


Social Studies, 12.01.2021 01:30

Mathematics, 12.01.2021 01:30

English, 12.01.2021 01:30

Medicine, 12.01.2021 01:30


Geography, 12.01.2021 01:30



Biology, 12.01.2021 01:40

Biology, 12.01.2021 01:40

Mathematics, 12.01.2021 01:40

Mathematics, 12.01.2021 01:40

Chemistry, 12.01.2021 01:40

History, 12.01.2021 01:40


Chemistry, 12.01.2021 01:40

Mathematics, 12.01.2021 01:40

Mathematics, 12.01.2021 01:40

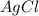 is added.
is added.


