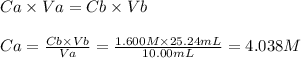Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
Calculate the molarity of an hcl solution if a 10.00 ml sample requires 25.24 ml of a 1.600 m naoh s...
Questions


Spanish, 09.02.2021 19:10

Mathematics, 09.02.2021 19:10

English, 09.02.2021 19:10

Mathematics, 09.02.2021 19:10

Mathematics, 09.02.2021 19:10

History, 09.02.2021 19:10



Arts, 09.02.2021 19:10

Mathematics, 09.02.2021 19:10

Mathematics, 09.02.2021 19:10


Mathematics, 09.02.2021 19:10


Mathematics, 09.02.2021 19:10

Chemistry, 09.02.2021 19:10

Mathematics, 09.02.2021 19:10

Mathematics, 09.02.2021 19:10

Mathematics, 09.02.2021 19:10