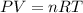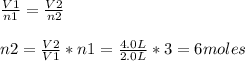Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle. if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3.2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
Assuming constant pressure and temperature, how many moles of gas have been added to the initial 3 m...
Questions

Social Studies, 14.12.2021 01:20



Mathematics, 14.12.2021 01:20

Chemistry, 14.12.2021 01:20

History, 14.12.2021 01:20

Mathematics, 14.12.2021 01:20

Spanish, 14.12.2021 01:20

Mathematics, 14.12.2021 01:20

Health, 14.12.2021 01:20




SAT, 14.12.2021 01:20


Computers and Technology, 14.12.2021 01:20

Social Studies, 14.12.2021 01:20


Chemistry, 14.12.2021 01:20