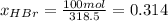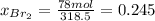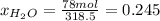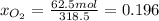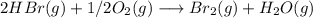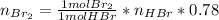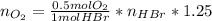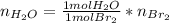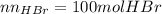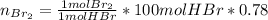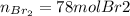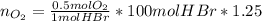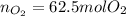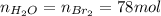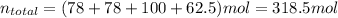Chemistry, 23.11.2019 04:31 rodrickahammonds
Bromine (br2) is produced by reacting hbr with o2, with water as a byproduct. the o2 is part of an air (21 mol % o2, 79 mol % n2) feed stream that is flowing sufficiently fast to provide 25% excess oxygen ("excess" has a precise meaning in process analysis: in this case there is 25% more oxygen than the amount needed to completely react with the limiting reactant). the fractional conversion of hbr is 78%.
a) show the degree of freedom analysis. be as specific as possible about labeling the unknowns and completely write out all of the independent equations.
b) calculate the composition (mole fractions) of the product stream.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
Bromine (br2) is produced by reacting hbr with o2, with water as a byproduct. the o2 is part of an a...
Questions




Mathematics, 24.01.2020 19:31


Chemistry, 24.01.2020 19:31




English, 24.01.2020 19:31

Mathematics, 24.01.2020 19:31

Mathematics, 24.01.2020 19:31


Mathematics, 24.01.2020 19:31



History, 24.01.2020 19:31



Computers and Technology, 24.01.2020 19:31