
Naoh(s) dissolves readily in water to form na+(aq) and oh-(aq). if 35.1 g of naoh is dissolved in 199 g of water in a coffee cup calorimeter, the temperature of the resulting solution increases from 23.0 oc to 62.6 oc. calculate the enthalpy change per mole of naoh dissolved. assume the specific heat capacity of the solution is 4.18 j/oc*g.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Hannah is writing a report on how albedo affects the global climate. she’s proofreading her passage for any factual errors. which sentence must hannah correct before submitting her report? earth receives energy from the sun. this energy drives many of the processes on earth, including its climate. some part of this energy is reflected by earth’s surface. we use the term albedo to describe the reflected energy. albedo of an object is the ratio of the reflected radiation to the total radiation reaching the object. a value of 0 means no energy is absorbed by the object, whereas a value of 1 means that all of the energy is absorbed. in this way, the albedo of an object can influence earth’s atmospheric temperature.
Answers: 1

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Naoh(s) dissolves readily in water to form na+(aq) and oh-(aq). if 35.1 g of naoh is dissolved in 19...
Questions



Biology, 20.03.2020 02:57




Biology, 20.03.2020 02:57


Mathematics, 20.03.2020 02:57

Mathematics, 20.03.2020 02:57

Mathematics, 20.03.2020 02:57



Mathematics, 20.03.2020 02:57

Mathematics, 20.03.2020 02:57



History, 20.03.2020 02:57

Mathematics, 20.03.2020 02:57

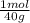 = 0,878 moles
= 0,878 moles



