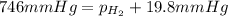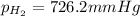Chemistry, 23.11.2019 05:31 Simplytaylorgrenade
Areaction of 0.028 g of magnesium with excess hydrochloric acid generated 31.0 ml of hydrogen gas. the gas was collected by water displacement in a 22 °c water bath. the barometric pressure in the lab that day was 746 mm hg. use dalton's law to calculate the partial pressure of hydrogen gas in the gas-collecting tube.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1


Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
Areaction of 0.028 g of magnesium with excess hydrochloric acid generated 31.0 ml of hydrogen gas. t...
Questions


Mathematics, 26.04.2021 23:10

English, 26.04.2021 23:10


Mathematics, 26.04.2021 23:10

Mathematics, 26.04.2021 23:10

Mathematics, 26.04.2021 23:10

Mathematics, 26.04.2021 23:10


Mathematics, 26.04.2021 23:10

Spanish, 26.04.2021 23:10


Mathematics, 26.04.2021 23:10

Mathematics, 26.04.2021 23:10

Arts, 26.04.2021 23:10


Mathematics, 26.04.2021 23:10



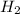 is, 726.2 mmHg
is, 726.2 mmHg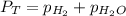
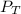 = total partial pressure = barometric pressure = 746 mmHg
= total partial pressure = barometric pressure = 746 mmHg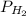 = partial pressure of hydrogen gas = ?
= partial pressure of hydrogen gas = ?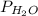 = partial pressure of water vapor = 19.8 mmHg (assume)
= partial pressure of water vapor = 19.8 mmHg (assume)