Chemistry, 23.11.2019 21:31 michelle453
For the reaction, 2a + b → 3c, rate of disappearance of a is 0.3 m/s in a certain time interval. what is the average rate of appearance of c and what is the average rate of the reaction in that time interval?
a) rate of appearance of c = 0.45 m/s
rate of the reaction = 0.6 m/s
b) rate of appearance of c = 0.2 m/s
rate of the reaction = 0.6 m/s
c) rate of appearance of c = 0.2 m/s
rate of the reaction = 0.15 m/s
d) rate of appearance of c = 0.45 m/s
rate of the reaction = 0.3 m/s
e) rate of appearance of c = 0.45 m/s
rate of the reaction = 0.15 m/s

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1


Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

You know the right answer?
For the reaction, 2a + b → 3c, rate of disappearance of a is 0.3 m/s in a certain time interval. wha...
Questions

History, 19.04.2020 04:48

Mathematics, 19.04.2020 04:48

Biology, 19.04.2020 04:48





Social Studies, 19.04.2020 04:48

Mathematics, 19.04.2020 04:48

Mathematics, 19.04.2020 04:48



Mathematics, 19.04.2020 04:48


Health, 19.04.2020 04:48

Social Studies, 19.04.2020 04:48



Mathematics, 19.04.2020 04:49

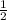
![\frac{d[A]}{dt}](/tpl/images/0388/2473/59baa.png) = -
= -![\frac{d[B]}{dt}](/tpl/images/0388/2473/a0d46.png) =
= 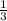
![\frac{d[C]}{dt}](/tpl/images/0388/2473/484ab.png) → equation 1
→ equation 1