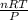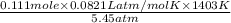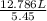Chemistry, 17.12.2019 04:31 khikhi1705
What would be the volume in liters of an ideal gas, if a 0.111 mole sample of the gas had a temperature of 1130 degrees celsius at a pressure of 5.45 atmospheres? (the ideal gas constant is 0.0821 l•atm/mol•k.)
0.43 liters
1.43 liters
1.89 liters
2.35 liters

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1


Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
What would be the volume in liters of an ideal gas, if a 0.111 mole sample of the gas had a temperat...
Questions


Biology, 06.08.2019 18:30












Mathematics, 06.08.2019 18:30






Mathematics, 06.08.2019 18:30