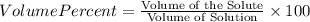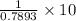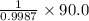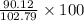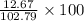Chemistry, 24.11.2019 12:31 Samanthas6365
Find both the volume percent of a solution that has 10.0 g of ethanol (d = 0.7893 g/ml) and 90.0 g of water (d = 0.9987 g/ml). assume volumes are additive.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

You know the right answer?
Find both the volume percent of a solution that has 10.0 g of ethanol (d = 0.7893 g/ml) and 90.0 g o...
Questions


Mathematics, 24.09.2020 03:01

Mathematics, 24.09.2020 03:01

Spanish, 24.09.2020 03:01

History, 24.09.2020 03:01

Computers and Technology, 24.09.2020 03:01



Mathematics, 24.09.2020 03:01



Mathematics, 24.09.2020 03:01




History, 24.09.2020 03:01

Physics, 24.09.2020 03:01



Mathematics, 24.09.2020 03:01