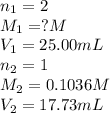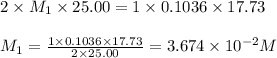Chemistry, 25.11.2019 19:31 michellectucker1982
Astudent titrated a 25.00-ml sample of a solution containing an unknown weak, diprotic acid (h2a) with n2oh. if the titration required 17.73 ml of 0.1036 m n2oh to completely neutralize the acid, calculate the concentration (in m) of the weak acid in the sample.
(a) 9.184 x 10 m
(b) 3.674 x 10-2 m
(c) 7.304 x 10-2 m
(d) 7.347 x 10-2 m
(e) 1.469 x 101 m

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1

Chemistry, 23.06.2019 14:00
How can a ringing telephone can be heard through a closed door
Answers: 1
You know the right answer?
Astudent titrated a 25.00-ml sample of a solution containing an unknown weak, diprotic acid (h2a) wi...
Questions


Mathematics, 04.09.2020 05:01


Mathematics, 04.09.2020 05:01

Mathematics, 04.09.2020 05:01


Mathematics, 04.09.2020 05:01




Arts, 04.09.2020 05:01

Business, 04.09.2020 05:01

Mathematics, 04.09.2020 05:01


Chemistry, 04.09.2020 05:01

History, 04.09.2020 05:01



History, 04.09.2020 05:01

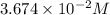
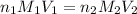
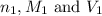 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 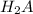
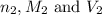 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.