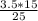Chemistry, 25.11.2019 19:31 avriannamcclenton1
If 25 ml of koh were needed to neutralize 15 ml of 3.5 m hbr, calculate the molarity of the base

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
If 25 ml of koh were needed to neutralize 15 ml of 3.5 m hbr, calculate the molarity of the base...
Questions



Chemistry, 05.05.2021 20:20


Biology, 05.05.2021 20:20

Mathematics, 05.05.2021 20:20




Mathematics, 05.05.2021 20:20

Mathematics, 05.05.2021 20:20



Mathematics, 05.05.2021 20:20


Mathematics, 05.05.2021 20:20


Mathematics, 05.05.2021 20:20

Mathematics, 05.05.2021 20:20

Mathematics, 05.05.2021 20:20

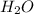
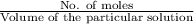
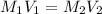 ------------------------------(1)
------------------------------(1)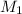 = Molarity of Acid
= Molarity of Acid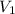 = Volume of Acid
= Volume of Acid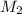 = Molarity of Base
= Molarity of Base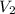 = Volume of Base
= Volume of Base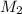 × 25
× 25