
Chemistry, 25.11.2019 20:31 jakobrobinette
Kemmi major does some experimental work on the combustion of sucrose: c12h22o11(s) 12 o2(g) → 12 co2(g) 11 h2o(g) she burns a 0.05392 g pellet of sucrose in a bomb calorimeter with excess oxygen. she determines the qrxn to be –916.6 j for the reaction. calculate the ∆h value for the combustion reaction. (round the answer to 3 significant digits, units of kj, pay attention to positive or negative.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Kemmi major does some experimental work on the combustion of sucrose: c12h22o11(s) 12 o2(g) → 12 co...
Questions



Social Studies, 25.09.2019 00:30


Physics, 25.09.2019 00:30

History, 25.09.2019 00:30

Health, 25.09.2019 00:30

Social Studies, 25.09.2019 00:30

Mathematics, 25.09.2019 00:30


Health, 25.09.2019 00:30


Mathematics, 25.09.2019 00:30


English, 25.09.2019 00:30

Mathematics, 25.09.2019 00:30


Health, 25.09.2019 00:30


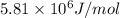
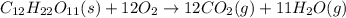
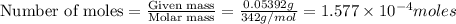
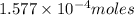 of sucrose releases = 916.6 J of heat
of sucrose releases = 916.6 J of heat
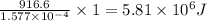 of heat
of heat


