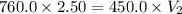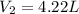Chemistry, 25.11.2019 20:31 bloodyflower2003
The manufacturer specs for a particular balloon indicate the maximum inflated volume is 3.00 l. the balloon is filled with 2.50 l of helium at sea level (assume p= 1.00 atm) and released. when the balloon rises to a higher altitude where the pressure is 450.0 mm hg, will the balloon burst? show a calculation to support your answer. assume constant temperature.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2
You know the right answer?
The manufacturer specs for a particular balloon indicate the maximum inflated volume is 3.00 l. the...
Questions



History, 01.06.2021 03:00


Mathematics, 01.06.2021 03:00


Medicine, 01.06.2021 03:00





Mathematics, 01.06.2021 03:00

Mathematics, 01.06.2021 03:00


Mathematics, 01.06.2021 03:00


Biology, 01.06.2021 03:00



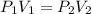
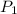 = initial pressure of gas= 1.00 atm = 760.0 mm Hg
= initial pressure of gas= 1.00 atm = 760.0 mm Hg 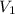 = initial volume of gas = 2.50 L
= initial volume of gas = 2.50 L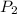 = final pressure of gas= 450.0 mm Hg
= final pressure of gas= 450.0 mm Hg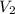 = final volume of gas = ?
= final volume of gas = ?