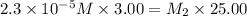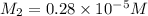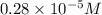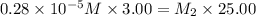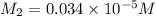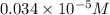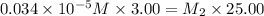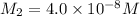Chemistry, 25.11.2019 20:31 monicaharris3
Suppose you start with a solution of red dye #40 that is 2.3 ✕ 10−5 m. if you do three successive volumetric dilutions pipetting 3.00 ml of solution and diluting with water in a 25.00 ml volumetric flask, what is the molarity of the final dilution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1

Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1
You know the right answer?
Suppose you start with a solution of red dye #40 that is 2.3 ✕ 10−5 m. if you do three successive vo...
Questions


Mathematics, 10.12.2020 23:20


English, 10.12.2020 23:20




Mathematics, 10.12.2020 23:20


Mathematics, 10.12.2020 23:20



Social Studies, 10.12.2020 23:20


Mathematics, 10.12.2020 23:20



Mathematics, 10.12.2020 23:20


English, 10.12.2020 23:20

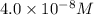
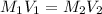
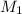 = molarity of stock solution =
= molarity of stock solution = 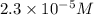
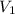 = volume of stock solution = 3.00 ml
= volume of stock solution = 3.00 ml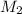 = molarity of diluted solution = ?
= molarity of diluted solution = ?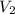 = volume of diluted solution = 25.00 ml
= volume of diluted solution = 25.00 ml