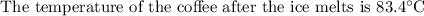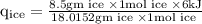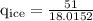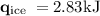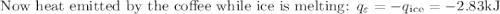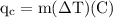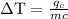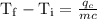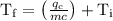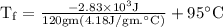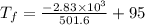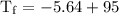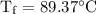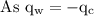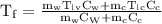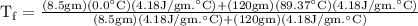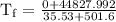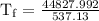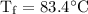Chemistry, 25.11.2019 21:31 jenorajordan5387
An ice cube of mass 8.5g is added to a cup of coffee, whose temperature is 95 degrees celcius and which contains 120 g of liquid. assume the specific heat capacity of coffee is the same as that of water. the heat of fusion of the ice (the heat associated with ice melting) is 6.0 kj/mol. find the temperature of the coffee after the ice melts.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1

Chemistry, 23.06.2019 13:30
32p and 31p are two isotopes of phosphorus. compare the number if subatomic particles that are present in the atoms of these isotopes.
Answers: 1

You know the right answer?
An ice cube of mass 8.5g is added to a cup of coffee, whose temperature is 95 degrees celcius and wh...
Questions




History, 11.03.2021 03:20


Mathematics, 11.03.2021 03:20


Mathematics, 11.03.2021 03:20



Computers and Technology, 11.03.2021 03:20


History, 11.03.2021 03:20


English, 11.03.2021 03:20

Mathematics, 11.03.2021 03:20



Mathematics, 11.03.2021 03:20