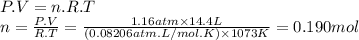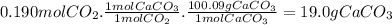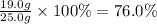Chemistry, 25.11.2019 21:31 zanaplen27
For the reaction below, kp = 1.16 at 800.°c. caco3(s) equilibrium reaction arrow cao(s) + co2(g) if a 25.0-g sample of caco3 is put into a 14.4 l container and heated to 800°c, what percentage by mass of the caco3 will react to reach equilibrium?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
For the reaction below, kp = 1.16 at 800.°c. caco3(s) equilibrium reaction arrow cao(s) + co2(g) if...
Questions

Chemistry, 03.12.2020 23:50

Engineering, 03.12.2020 23:50



Mathematics, 03.12.2020 23:50




Biology, 03.12.2020 23:50

Mathematics, 03.12.2020 23:50

Mathematics, 03.12.2020 23:50


English, 03.12.2020 23:50


History, 03.12.2020 23:50


English, 03.12.2020 23:50

Mathematics, 03.12.2020 23:50


Mathematics, 03.12.2020 23:50