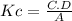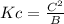Chemistry, 25.11.2019 21:31 FrankyV3220
Write an equilibrium expression for each chemical equation involving one or more solid or liquid reactants or products. part a: hcho2(aq)+h2o(l)⇌h3o+(aq)+cho−2(aq) use a for [hcho2], b for [h2o], c for [h3o+], d for [cho−2].
part b: co2−3(aq)+h2o(l)⇌hco−3(aq)+oh−(aq) use a for [co2−3], b for [h2o], c for [hco−3], d for [oh−].
part c: 2c(s)+o2(g)⇌2co(g) use a for [c], b for [o2], c for [co].
part d: c(s)+co2(g)⇌2co(g) use a for [c], b for [co2], c for [co].

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 03:30
Ineed pls urgent 1-20 in order and fully detail step my step.
Answers: 1
You know the right answer?
Write an equilibrium expression for each chemical equation involving one or more solid or liquid rea...
Questions

Mathematics, 26.03.2021 19:50





Mathematics, 26.03.2021 19:50



Mathematics, 26.03.2021 19:50


Arts, 26.03.2021 19:50




Mathematics, 26.03.2021 19:50

Physics, 26.03.2021 19:50


Biology, 26.03.2021 19:50