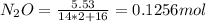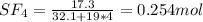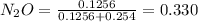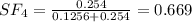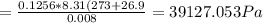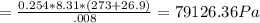A8.00 l tank at 26.9 c is filled with 5.53 g of dinitrogen difluoride gas and 17.3 g of sulfur hexafluoride gas. you can assume both gases behave as ideal gases under these conditions. calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which type of stress results when two plates push against one another? a. compression b. tension c. force d. shear
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2


You know the right answer?
A8.00 l tank at 26.9 c is filled with 5.53 g of dinitrogen difluoride gas and 17.3 g of sulfur hexaf...
Questions


Engineering, 16.02.2020 04:44

Mathematics, 16.02.2020 04:44

Mathematics, 16.02.2020 04:44

Mathematics, 16.02.2020 04:44

Mathematics, 16.02.2020 04:44

Mathematics, 16.02.2020 04:44


Chemistry, 16.02.2020 04:44

Computers and Technology, 16.02.2020 04:44


Chemistry, 16.02.2020 04:44

Biology, 16.02.2020 04:45

Mathematics, 16.02.2020 04:45


Advanced Placement (AP), 16.02.2020 04:46




Mathematics, 16.02.2020 04:47