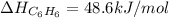Chemistry, 25.11.2019 23:31 ZeroFrost7899
Calculate δh∘f (in kilojoules per mole) for benzene, c6h6, from the following data: 2c6h6(l) + 15o2(g)→12co2(g)+6h2o(l) δh∘=−6534.0 kjδh∘f co2=−393.5 kj/molδh∘f h2o=−285.8 kj/mol express the enthalpy change in kilojoules per mole to three significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Zinc + lead(ii) nitrate yield zinc nitrate + leadwhat's the chemical equation for this?
Answers: 1

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1


Chemistry, 23.06.2019 10:30
Amethod of separation that employs a system with two phases of matter, a mobile phase and a stationary phase, is called
Answers: 2
You know the right answer?
Calculate δh∘f (in kilojoules per mole) for benzene, c6h6, from the following data: 2c6h6(l) + 15o2...
Questions


Mathematics, 31.08.2021 22:30






English, 31.08.2021 22:30

English, 31.08.2021 22:30

Mathematics, 31.08.2021 22:30

Mathematics, 31.08.2021 22:30

Mathematics, 31.08.2021 22:30

Business, 31.08.2021 22:40

English, 31.08.2021 22:40

Mathematics, 31.08.2021 22:40





Geography, 31.08.2021 22:40

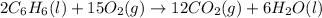
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0390/4702/76c37.png)
![\Delta H=[(n_{CO_2}\times \Delta H_{CO_2})+(n_{H_2O}\times \Delta H_{H_2O})]-[(n_{O_2}\times \Delta H_{O_2})+(n_{C_6H_6}\times \Delta H_{C_6H_6})]](/tpl/images/0390/4702/15aa8.png)
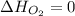 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero![-6534.0=[(12\times -393.5)+(6\times -285.8)]-[(15\times 0)+(2\times \Delta H_{C_6H_6})]](/tpl/images/0390/4702/ed6d2.png)