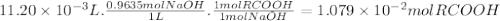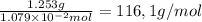Chemistry, 25.11.2019 23:31 golffuture666
What is the molecular weight of a monoprotic carboxylic acid if 11.20 ml of 0.9635 m sodium hydroxide is required to titrate 1.253 g of this acid? the reaction is represented by following equation. note: in the equation, r represents an unspecified carbon containing structure rco-h (aq)naoh (aq) rco, na (aq) hoh (u) separate experiments suggest the unknown acid is likely pentanoic acid, c4h, co2h. is the unknown pentanoic acid? why or why not?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1


Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
What is the molecular weight of a monoprotic carboxylic acid if 11.20 ml of 0.9635 m sodium hydroxid...
Questions


History, 21.11.2020 08:00

Mathematics, 21.11.2020 08:00

Social Studies, 21.11.2020 08:00

Mathematics, 21.11.2020 08:00

Physics, 21.11.2020 08:00

English, 21.11.2020 08:00


Mathematics, 21.11.2020 08:00

Mathematics, 21.11.2020 08:00



Mathematics, 21.11.2020 08:00


Mathematics, 21.11.2020 08:00


Health, 21.11.2020 08:00

Mathematics, 21.11.2020 08:00


Biology, 21.11.2020 08:00