Chemistry, 26.11.2019 01:31 billlyyyyyyyyyy
Calculate the change internal energy (δe) for a system that is giving off 65.0 kj of heat and is performing 855 j of work on the surroundings. calculate the change internal energy (δe) for a system that is giving off 65.0 kj of heat and is performing 855 j of work on the surroundings. 9.00 x 102 kj 64.1 kj -9.00 x 102 kj -64.1 kj -65.9 kj

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1
You know the right answer?
Calculate the change internal energy (δe) for a system that is giving off 65.0 kj of heat and is per...
Questions

Chemistry, 01.12.2020 23:40

Mathematics, 01.12.2020 23:40

Mathematics, 01.12.2020 23:40


Social Studies, 01.12.2020 23:40




English, 01.12.2020 23:40

Mathematics, 01.12.2020 23:40



Mathematics, 01.12.2020 23:40




Geography, 01.12.2020 23:40


Mathematics, 01.12.2020 23:40

Chemistry, 01.12.2020 23:40

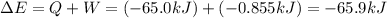
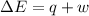
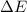 =Change in internal energy
=Change in internal energy
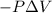 {Work is done by the system is negative as the final volume is greater than initial volume}
{Work is done by the system is negative as the final volume is greater than initial volume}