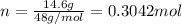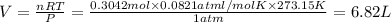Chemistry, 26.11.2019 01:31 rafexRaex2914
The effect of chlorofluorocarbons (such as ccl2f2(g)) on the depletion of the ozone layer is well known. the use of substitutes, such as ch3ch2f(g), for the chlorofluorocarbons, has largely corrected the problem. calculate the volume (in l) occupied by 14.6 g of each of these compounds at stp. (a) ccl2f2(g) l (b) ch3ch2f(g) l

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
The effect of chlorofluorocarbons (such as ccl2f2(g)) on the depletion of the ozone layer is well kn...
Questions

Mathematics, 09.12.2020 19:20


History, 09.12.2020 19:20




History, 09.12.2020 19:20





Spanish, 09.12.2020 19:20

Arts, 09.12.2020 19:20

English, 09.12.2020 19:20

Chemistry, 09.12.2020 19:20





Social Studies, 09.12.2020 19:20

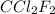 at STP = n
at STP = n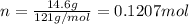
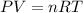 (ideal gas equation)
(ideal gas equation)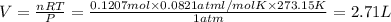
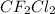 is 2.71 liters.
is 2.71 liters.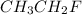 at STP = n
at STP = n