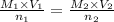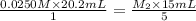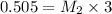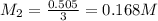Ferris & mona used the orp sensor to titrate a ferrous ammonium sulfate solution, (nh4)2fe(so4)2 with kmno4 titrant.
they titrated a 15.00 ml aliquot of the fe+2 solution with 0.0250 m mno4- solution and determined that the equivalence point was at 20.2 ml.
what is the molarity of the fe+2 solution? 5 fe+2(aq) + mno4-(aq) + 8 h+(aq) â 5 fe+3(aq) + mn+2(aq) + 4 h2oselect one: a. 0.168 mb. 0.0928 mc. 0.0337 md. 0.673 m

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1


Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
Ferris & mona used the orp sensor to titrate a ferrous ammonium sulfate solution, (nh4)2fe(so4)...
Questions

Mathematics, 03.02.2021 07:40

Business, 03.02.2021 07:40


English, 03.02.2021 07:40





English, 03.02.2021 07:40

Mathematics, 03.02.2021 07:40

Social Studies, 03.02.2021 07:40

Mathematics, 03.02.2021 07:40

Mathematics, 03.02.2021 07:40

History, 03.02.2021 07:40


Mathematics, 03.02.2021 07:40


English, 03.02.2021 07:40

English, 03.02.2021 07:40

Computers and Technology, 03.02.2021 07:40