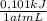Chemistry, 26.11.2019 03:31 shelbylynn737
Find δe° for the reaction below if the process is carried out at a constant pressure of 1.00 atm andδv (the volume change) = -24.5 l. (1 l ∙ atm = 101 j)
2 co(g) + o2 (g) → 2 co2(g) δh° = -566. kj
a. +2.47 kj
b. -568 kj
c. -2.47 kj
d. -564 kj

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3


You know the right answer?
Find δe° for the reaction below if the process is carried out at a constant pressure of 1.00 atm and...
Questions

English, 05.07.2019 00:30




English, 05.07.2019 00:30



Social Studies, 05.07.2019 00:30


Social Studies, 05.07.2019 00:30



Chemistry, 05.07.2019 00:30

Chemistry, 05.07.2019 00:30

Physics, 05.07.2019 00:30

Social Studies, 05.07.2019 00:30

Chemistry, 05.07.2019 00:30

English, 05.07.2019 00:30

Chemistry, 05.07.2019 00:30