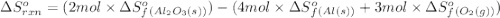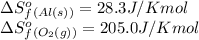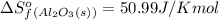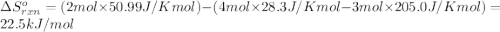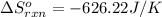Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1

Chemistry, 23.06.2019 01:30
List and describe the neurological effects of the vocs and other air pollutants,as described by dr.theo colborn
Answers: 2

Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
You know the right answer?
Aluminum forms a layer of aluminum oxide when exposed to air which protects the bulk metal from furt...
Questions

Computers and Technology, 25.01.2020 03:31


Health, 25.01.2020 03:31

Mathematics, 25.01.2020 03:31

Social Studies, 25.01.2020 03:31


Geography, 25.01.2020 03:31


English, 25.01.2020 03:31






Mathematics, 25.01.2020 03:31


Mathematics, 25.01.2020 03:31

English, 25.01.2020 03:31


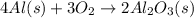
![\Delta S^o_{rxn}=\sum [n\times \Delta S^o_f(product)]-\sum [n\times \Delta S^o_f(reactant)]](/tpl/images/0390/9147/5a45f.png)