
Chemistry, 26.11.2019 03:31 aghamuzahirali5289
Aphosphate buffer solution (25.00 ml sample) used for a growth medium was titrated with 0.1000 m hydrochloric acid. the components of the buffer were sodium monohydrogenphosphate and sodium dihydrogenphosphate. the first endpoint occurred at a volume of 10.32 ml, and the second occurred after an additional 18.62 ml was added, for a total volume of 28.94 ml. what was the total concentration of phosphate (in any form) in the buffer?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

You know the right answer?
Aphosphate buffer solution (25.00 ml sample) used for a growth medium was titrated with 0.1000 m hyd...
Questions

Mathematics, 23.11.2020 02:10

Mathematics, 23.11.2020 02:10

English, 23.11.2020 02:10

English, 23.11.2020 02:10





Mathematics, 23.11.2020 02:10





Biology, 23.11.2020 02:20

Mathematics, 23.11.2020 02:20



Social Studies, 23.11.2020 02:20

Health, 23.11.2020 02:20

Mathematics, 23.11.2020 02:20

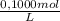 = 1,862x10⁻³moles of HCl ≡ moles of phosphate buffer.
= 1,862x10⁻³moles of HCl ≡ moles of phosphate buffer.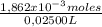 = 0,07448M of phosphate buffer
= 0,07448M of phosphate buffer

