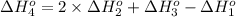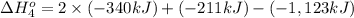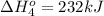Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2


Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1
You know the right answer?
Calculate the enthalpy for this reaction: 2c(s) + h2(g) > c2h2(g) δh° = kj given the following...
Questions


Computers and Technology, 15.02.2021 01:00


Mathematics, 15.02.2021 01:00

Mathematics, 15.02.2021 01:00



Computers and Technology, 15.02.2021 01:00


Mathematics, 15.02.2021 01:00

Mathematics, 15.02.2021 01:00

Arts, 15.02.2021 01:00







Mathematics, 15.02.2021 01:00

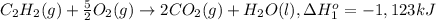 ...[1]
...[1]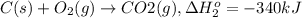 ..[2]
..[2]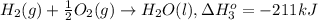 ..[3]
..[3]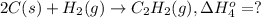 ..[4]
..[4]