
Chemistry, 26.11.2019 04:31 Imsofrie8111
Achemistry student is given 3.00 l of a clear aqueous solution at 17.° c . he is told an unknown amount of a certain compound x is dissolved in the solution. the student allows the solution to cool to 17.° c . the solution remains clear. he then evaporates all of the water under vacuum. a precipitate remains. the student washes, dries and weighs the precipitate. it weighs 0.15 kg . using the above information can you calculate the solubility, x, in water at 17 degrees c

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3


Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2
You know the right answer?
Achemistry student is given 3.00 l of a clear aqueous solution at 17.° c . he is told an unknown amo...
Questions


History, 19.12.2019 06:31





History, 19.12.2019 06:31





Chemistry, 19.12.2019 06:31

Biology, 19.12.2019 06:31

English, 19.12.2019 06:31




Mathematics, 19.12.2019 06:31

Biology, 19.12.2019 06:31

Social Studies, 19.12.2019 06:31

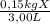 = 0,05kg/L
= 0,05kg/L

